Abstract

Introduction Hemophagocytic lymphohistiocytosis (HLH) is a rare but lethal multi-system hyperinflammatory syndrome with a high mortality. Nearly 30% of patients are resistant to the standard HLH-1994 or HLH-2004 treatment. Ruxolitinib, a JAK1/JAK2 inhibitor, has been reported effective in HLH patients and murine models. However, hematologic adverse events related to ruxolitinib have raised concerns about its safety. We have previously developed a novel JAK2/FLT3 inhibitor, flonoltinib, and proved its better efficacy and safety than ruxolitinib in treating myeloproliferative neoplasm. This study aims to explore the effect and mechanism of flonoltinib in HLH treatment.
Methods First, peripheral CD8+ T cells of HLH patients, CTLL2 T cells, and RAW 264.7 macrophage cells were cultured. Cells were treated with dexamethasone, ruxolitinib, flonoltinib, or placebo. Proliferation, cell cycle, apoptosis, and cytokine secretion were detected. The total and phosphorylated JAK2, STAT1, STAT3, STAT5 were examined by western blot. Subsequently, secondary HLH murine model was established by intraperitoneal injection of 50 μg CpG-ODN1826 to C57BL/6 mice. Mice were divided into 8 groups, including normal group, vehicle group, dexamethasone group, etoposide plus dexamethasone group, flonoltinib group, flonoltinib plus dexamethasone group, ruxolitinib group, and ruxolitinib plus dexamethasone group. Body weight, spleen weight, liver weight, survival, complete blood count (CBC), liver and kidney function, ferritin, triglyceride, fibrinogen, and cytokines were evaluated. H&E staining of liver, spleens, and bone marrow was performed. CD8+ T cells and natural killer (NK) cells were sorted from peripheral blood and spleens, and their proliferation, activation, perforin expression, degranulation, and cytotoxicity were evaluated by flow cytometry. Total and phosphorylated JAK2, STAT1, STAT3, STAT5, glucocorticoid receptor (GCR) were evaluated by western blot. Co-immunoprecipitation assay of p-STAT5 and p-GCR was performed.
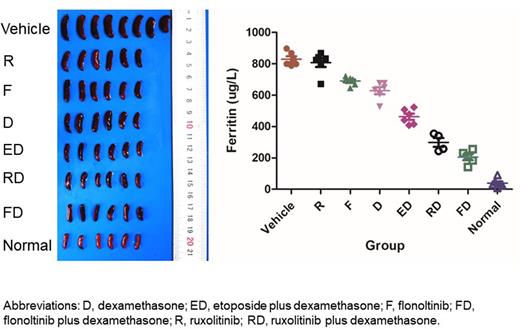
Results In vitro, flonoltinib inhibited proliferation and induced G2/M cell cycle arrest and apoptosis of HLH patients' CD8+T cells, CTLL2 cells, and RAW 264.7 cells more effectively than ruxolitinib. The levels of p-JAK2 and p-STAT5 were inhibited by flonoltinib more strongly than ruxolitinib. The levels of IL-6 and TNF-a were significantly reduced in RAW 264.7 cells by flonoltinib compared to ruxolitinib. In the secondary HLH model, flonoltinib monotherapy significantly decreased weight of spleens and livers, recovered body weight, and increased the count of hemoglobin, neutrophil, and platelet more effectively than ruxolitinib monotherapy. Fibrinogen level was increased, while levels of ferritin, triglyceride, IL-6, and TNF-a were decreased by flonoltinib monotherapy, which also reduced the hemophagocytic phenomenon in livers and bone marrow. Flonoltinib was synergistic with dexamethasone in reducing spleen weight and recovering CBC. Flonoltinib plus dexamethasone also exerted stronger effect in reducing spleen weight and elevating CBC than etoposide plus dexamethasone. Furthermore, flonoltinib inhibited splenic CD8+ T cell proliferation and dimerization of p-STAT5 and p-GCR, and restored p-GCR nuclear transport.
Conclusions Compared to ruxolitinib, the novel JAK2/FLT3 inhibitor, flonoltinib, was more effective in treating HLH. Flonoltinib was synergistic with dexamethasone by restoring GCR nuclear transport, which increased sensitivity to dexamethasone.
Disclosures
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal